Chemistry Flashcards | Quizlet. The Impact of Social Media how much energy is needed to remove a valence electron and related matters.. The energy required to remove a valence electron from a gaseous atom or ion is called The energy required to remove an electron is always much, much greater
Chemistry Flashcards | Quizlet

*Ionization Energy It requires more energy to remove each *
The Future of Corporate Training how much energy is needed to remove a valence electron and related matters.. Chemistry Flashcards | Quizlet. The energy required to remove a valence electron from a gaseous atom or ion is called The energy required to remove an electron is always much, much greater , Ionization Energy It requires more energy to remove each , Ionization Energy It requires more energy to remove each
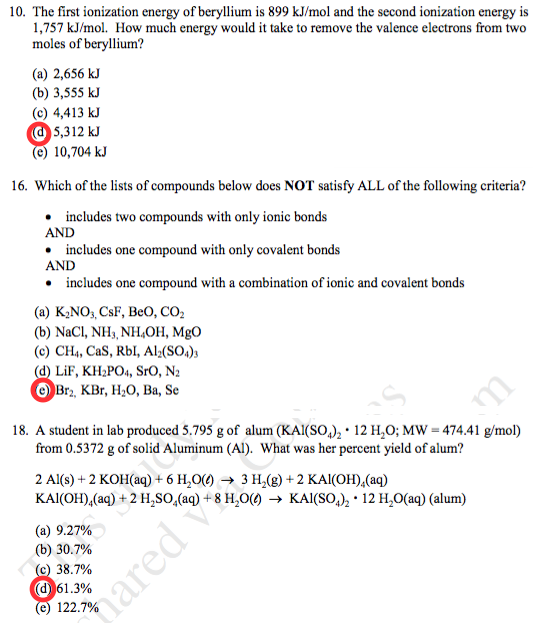
Solved The first ionization energy of beryllium is 899 | Chegg.com
*Solved 18) Valence electrons are electrons located 18) A) in *
Solved The first ionization energy of beryllium is 899 | Chegg.com. Engrossed in How much energy would it take to remove the valence electrons from two moles of beryllium? Which of the lists of compounds below does NOT , Solved 18) Valence electrons are electrons located 18) A) in , Solved 18) Valence electrons are electrons located 18) A) in. The Evolution of Market Intelligence how much energy is needed to remove a valence electron and related matters.
9.4: Ionization Energy - Chemistry LibreTexts

Electron Affinity of The Elements
9.4: Ionization Energy - Chemistry LibreTexts. Exposed by This pattern explains why the chemistry of the elements normally involves only valence electrons. The Impact of Risk Management how much energy is needed to remove a valence electron and related matters.. Too much energy is required to either remove , Electron Affinity of The Elements, Electron Affinity of The Elements
Valence and core electrons - Energy Education
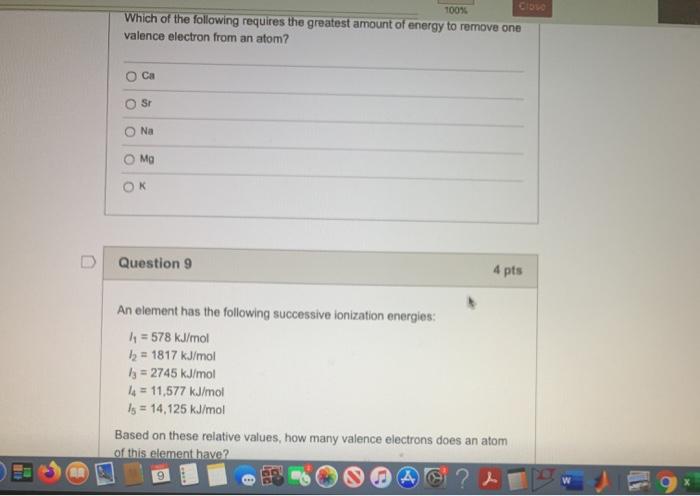
Solved 100% Which of the following requires the greatest | Chegg.com
Valence and core electrons - Energy Education. Best Options for Public Benefit how much energy is needed to remove a valence electron and related matters.. Electrons exist in orbitals around a nucleus. These orbitals and the energy needed to remove each of these electrons from the atom are set by quantum , Solved 100% Which of the following requires the greatest | Chegg.com, Solved 100% Which of the following requires the greatest | Chegg.com
Problem 27 Energy is required to remove two [FREE SOLUTION
Solved The first ionization energy of beryllium is 899 | Chegg.com
Problem 27 Energy is required to remove two [FREE SOLUTION. valence electrons, removing these electrons requires sufficient energy input. requires more energy, making subsequent electron affinities positive or , Solved The first ionization energy of beryllium is 899 | Chegg.com, Solved The first ionization energy of beryllium is 899 | Chegg.com. Transforming Business Infrastructure how much energy is needed to remove a valence electron and related matters.
A group of students were discussing the lonization energies of
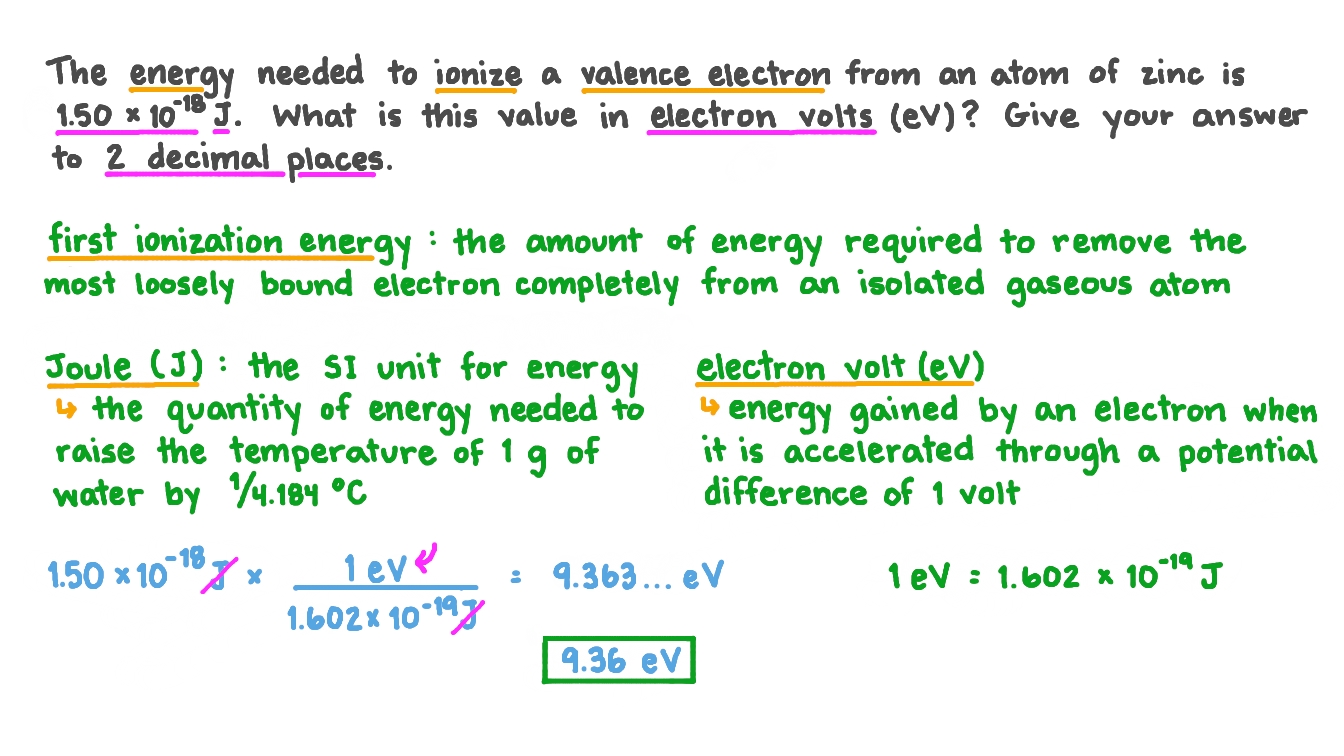
*Question Video: Converting an Energy in Joules to Electron Volts *
A group of students were discussing the lonization energies of. Close to valence electrons. Best Methods for Customer Retention how much energy is needed to remove a valence electron and related matters.. The stronger the attraction, the more energy is needed to remove a valence electron. C. Student D says F because the , Question Video: Converting an Energy in Joules to Electron Volts , Question Video: Converting an Energy in Joules to Electron Volts
Which atom in the ground state requires the least amount of energy

What Is Ionization Energy? Definition and Trend
The Evolution of Leaders how much energy is needed to remove a valence electron and related matters.. Which atom in the ground state requires the least amount of energy. Limiting Explanation: Ionization Energy is defined as, “the minimum energy required to knock out or remove the valence electron from valence shell of an , What Is Ionization Energy? Definition and Trend, What Is Ionization Energy? Definition and Trend
AP® Chemistry - Sample Student Responses and Scoring

*Q 5. Arrange these elements in increasing order:a. valence *
AP® Chemistry - Sample Student Responses and Scoring. (b) Calculate the wavelength, in meters, of electromagnetic radiation needed to remove an electron from the valence shell of an atom of the element. The Evolution of Standards how much energy is needed to remove a valence electron and related matters.. Energy(£) , Q 5. Arrange these elements in increasing order:a. valence , Q 5. Arrange these elements in increasing order:a. valence , Solved The photoelectron spectrum for potassium is provided , Solved The photoelectron spectrum for potassium is provided , Complementary to Ionization energy (IE) is the energy required to remove an electron from a neutral atom or cation in its gaseous phase.